TECNIS® Multifocal IOL +2.75 D
TECNIS® Multifocal IOL +2.75 D
Outstanding, Full-Range Vision* Tailored for Intermediate Distances
No two patients are exactly alike, which is why we offer multifocal lens options optimized for varying visual demands. The TECNIS® Multifocal IOL +2.75 D delivers a full range* of the high quality vision tailored for intermediate distances.

The TECNIS® Multifocal IOL +2.75 D is designed with your patients in mind. Optimized for those favoring intermediate vision activities like grocery shopping, it delivers tailored clarity at a theoretical reading distance of 50 cm.
Learn about multifocal lens options optimized for longer reading distances and near vision.
The TECNIS® Multifocal 1-Piece IOL features the only multifocal lenses capable of providing a full range* of high-quality vision (20/25 or better),1,2 tailored for each patient’s lifestyle.
- High quality vision across near, intermediate and distance vision*1,2
- Optimized for a theoretical reading distance of 50 cm with the +2.75 D lens1,2
Give your patients a lens designed for real-world performance. The TECNIS® Multifocal IOL +2.75 D delivers tailored increased spectacle independence in low light performance for outstanding outcomes, even at night.**1,2
How Often Do You Wear Glasses?**
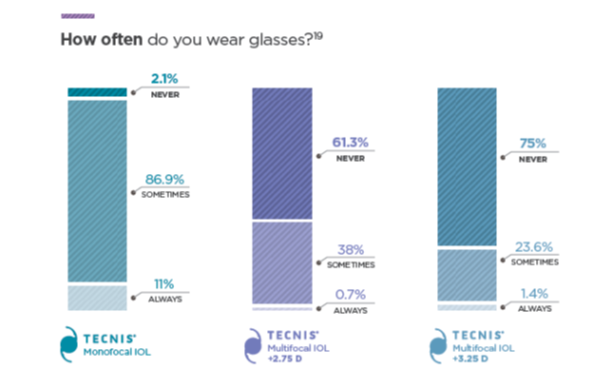
+4.0 D (purple) data are historical from a separate clinical study using the same test methodology.
Degree of Difficulty† With Night Vision**1,2
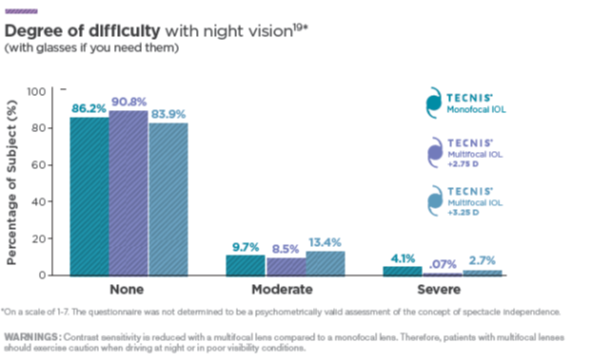
+4.0 D (purple) data are historical from a separate clinical study using the same test methodology.
Give your patients outstanding low-light performance:
- 90.8% of patients experienced no difficulty with night vision†1
- 76.8% of patients experienced no difficulty with glare†1
- 69.0% of patients experienced no difficulty with halos†1
Unlike another leading IOL, TECNIS® IOL material is not associated with glistenings.6 Glistenings cause light scatter.3-7
97% of patients would elect to have the TECNIS® Multifocal IOL +2.75 D again.**1
|
TECNIS® Multifocal IOL +2.75 D (ZKB00) |
||
|---|---|---|
|
OPTIC CHARACTERISTICS |
||
|
Powers: |
+5.0 D to 34.0 D in 0.5 diopter increments |
|
|
Diameter: |
6.0 mm |
|
|
Shape: |
Biconvex, anterior aspheric surface, posterior diffractive surface |
|
|
Add Power (IOL Plane): |
+2.75 D |
|
|
Add Power (Spec Plane): |
+2.01 D |
|
|
Material: |
UV-blocking hydrophobic acrylic |
|
|
Refractive Index: |
1.47 at 35˚ C |
|
|
Chromatic Aberration (Abbe Number): |
55 |
|
|
Edge Design: |
ProTEC frosted, continuous 360˚ posterior square edge |
|
|
BIOMETRY |
CONTACT ULTRASOUND |
OPTICAL |
|
A-Constant: |
118.8‡ |
119.3§ |
|
Theoretical AC Depth: |
5.40 mm |
5.72 mm |
|
Surgeon Factor:8 |
1.68 mm |
1.96 mm |
|
HAPTIC CHARACTERISTICS |
||
|
Overall Length: |
13.0 mm |
|
|
Style: |
C |
|
|
Material: |
UV-blocking hydrophobic acrylic |
|
|
Design: |
Haptics offset from optic |
|
View full product specifications (PDF)
IOL INSERTION
Recommended insertion instruments:
- UNFOLDER® Platinum 1 Series Delivery System: Intuitive push-and-twist, screw-style insertion designed to simplify lens implantation.
FOOTNOTES
*TECNIS Multifocal IOLs provide distance and near vision (ZLB00 and ZMB00) or distance and intermediate vision (ZKB00). In combination, the lenses can provide a full range of vision.
**The questionnaire was not determined to be a psychometrically valid assessment of the concept of spectacle independence.
†On a scale of 1-7, with glasses as needed
‡Value theoretically derived for a typical 20.00 D lens. Johnson & Johnson Vision recommends that surgeons personalize their A-constant based on their surgical techniques and equipment, experience with the lens model and postoperative results.
§Derived from clinical evaluation results of the TECNIS® 1-Piece Platform.
REFERENCES
1. TECNIS® Multifocal 1-Piece IOL DFU, Models ZKB00 and ZLB00. Santa Ana, Calif. Johnson & Johnson Surgical Vision, Inc.
2. TECNIS® Multifocal 1-Piece IOL DFU, Model ZMB00. Santa Ana, Calif. Johnson & Johnson Surgical Vision, Inc.
3. Nagata M, et al. Clinical evaluation of the transparency of hydrophobic acrylic intraocular lens optics. J Cataract Refract Surg. 2010;36(12):2056-2060.
4. Christiansen G, et al. Glistenings in the AcrySof® intraocular lens: Pilot study. J Cataract Refract Surg. 2001;27(5):728-733.
5. Colin J, et al. Incidence of glistenings with the latest generation of yellow-tinted hydrophobic acrylic intraocular lenses. J Cataract Refract Surg. 2012;38(7):1140-1146.
6. Gunenc U, et al. Effects on visual function of glistenings and folding marks in AcrySof® intraocular lenses. J Cataract Refract Surg. 2001;27(10):1611-1614.
7. Van der Mooren M, Franssen L, Piers P. Effects of glistenings in intraocular lenses. Biomed Opt Express. 2013;4(8):1294-1304.
8. Calculated based on Holladay I formula: Holladay JT, Prager TC, Chandler TY, Musgrove KH, Lewis JW, Ruis RS. A three-part system for refining intraocular lens power calculations. J Cataract Refract Surg. 1988;14(1)17-24
INDICATIONS AND IMPORTANT SAFETY INFORMATION FOR THE TECNIS® MULTIFOCAL 1-PIECE IOLS, MODELS ZKB00 AND ZLB00
Rx Only
INDICATIONS
The TECNIS® Multifocal 1-Piece Intraocular lenses, Models ZKB00 (+2.75 D) and ZLB00 (+3.25 D), are indicated for primary implantation for the visual correction of aphakia in adult patients with and without presbyopia in whom a cataractous lens has been removed by phacoemulsification and who desire near, intermediate, and distance vision with increased spectacle independence. The intraocular lenses are intended to be placed in the capsular bag.
WARNINGS
Physicians considering lens implantation should weight the potential risk/benefit ratio for any conditions described in the Directions for Use that could increase complications or impact patient outcomes. Multifocal IOL implants may be inadvisable in patients where central visual field reduction may not be tolerated, such as macular degeneration, retinal pigment epithelium changes, and glaucoma. The lens should not be placed in the ciliary sulcus. Inform patients about the possibility that a decrease in contrast sensitivity and an increase in visual disturbances may affect their ability to drive a car under certain environmental conditions, such as driving at night or in poor visibility conditions.
PRECAUTIONS
Prior to surgery, inform prospective patients of the possible risks and benefits associated with the use of this device and provide a copy of the patient information brochure to patient. The long term effects of intraocular lens implantation have not been determined. Secondary glaucoma has been reported occasionally in patients with controlled glaucoma who received lens implants. Do not reuse, resterilize or autoclave.
ADVERSE EVENTS
Only the rate (3.3%) of surgical re-interventions, most of which were non-lens-related, in the ZLB00 (+3.25 D) lens group, was statistically higher than the FDA grid rate (for both first and second eyes).
ATTENTION
Reference the Directions for Use for a complete listing of Indications and Important Safety Information.
INDICATIONS AND IMPORTANT SAFETY INFORMATION FOR TECNIS® MONOFOCAL 1-PIECE IOL
INDICATIONS
The TECNIS® 1-Piece lens is indicated for the visual correction of aphakia in adult patients in whom a cataractous lens has been removed by extracapsular cataract extraction. These devices are intended to be placed in the capsular bag.
WARNINGS
Physicians considering lens implantation should weigh the potential risk/benefit ratio for any conditions described in the TECNIS® 1-Piece IOL Directions for Use that could increase complications or impact patient outcomes. The TECNIS® 1-Piece IOL should not be placed in the ciliary sulcus.
PRECAUTIONS
Do not reuse, resterilize, or autoclave.
ADVERSE EVENTS
In 3.3% of patients, reported adverse events of cataract surgery with the TECNIS® 1-Piece IOL included macular edema.
ATTENTION
Reference the Directions for Use for a complete listing of indications and important safety information.
INDICATIONS AND IMPORTANT SAFETY INFORMATION FOR THE UNFOLDER® PLATINUM 1 SERIES IMPLANTATION SYSTEM
INDICATIONS
The Model DK7796 Handpiece is used in combination with the Model 1MTEC30 Cartridge to fold and assist in inserting Johnson & Johnson Surgical Vision Acrylic 1-Piece Intraocular Lenses, ONLY into the capsular bag.
CONTRAINDICATIONS
Do not use the handpiece if the rod tip appears nicked or damaged in any way.
WARNINGS
The UNFOLDER® Platinum 1 Series Implantation System should be used ONLY with JJSV Acrylic 1-Piece IOLs. Do not use if the cartridge tip is cracked or split prior to implantation. Never release the plunger until the optic body has been completely released from the cartridge tube.
See Full Indications and Important Safety Information.
ATTENTION
Reference the Directions for Use for a complete listing of Indications and Important Safety Information.
PP2021CT4044 V2.0

